October 29, 2007
Electron Carrier Molecules
So, in Mitochondria Pt. 2 I threw a whole lot of information at you, some of it without much context. In this post, I will hopefully provide some of that context. Specifically, we will explore the the different types of electron carrier molecules that are present in the electron transport chain.
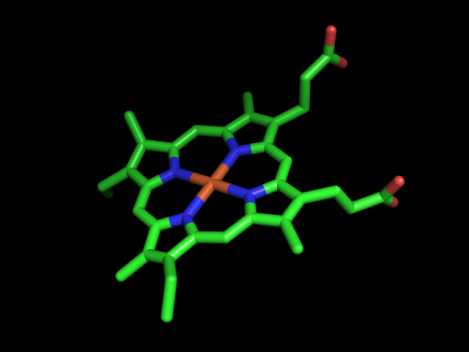 Most electron carrier molecules are proteins that have had electron accepting functional groups added to them. The cytochrome series of molecules are a good example of this. Cytochrome electron carriers all have a heme functional group added to them. The heme group from cytochrome c is imaged with pymol to the left. It is this heme group that allows the cytochrome carriers to accept the electrons that they carry–without such functional groups, the electron transport chain couldn’t work. If you are wondering where you might have heard of a heme group before, they are also present in molecules of hemoglobin, the protein that carries oxygen in the bloodstream.
Most electron carrier molecules are proteins that have had electron accepting functional groups added to them. The cytochrome series of molecules are a good example of this. Cytochrome electron carriers all have a heme functional group added to them. The heme group from cytochrome c is imaged with pymol to the left. It is this heme group that allows the cytochrome carriers to accept the electrons that they carry–without such functional groups, the electron transport chain couldn’t work. If you are wondering where you might have heard of a heme group before, they are also present in molecules of hemoglobin, the protein that carries oxygen in the bloodstream.
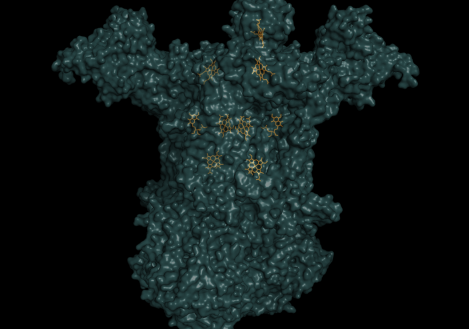
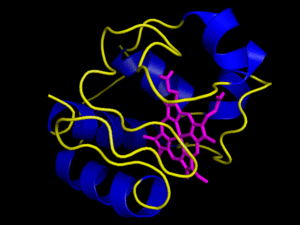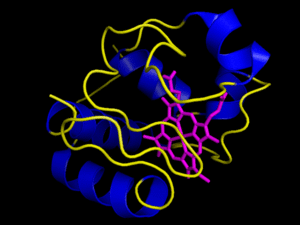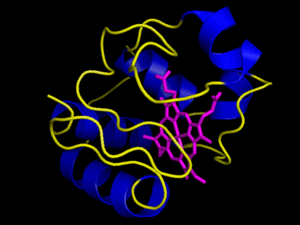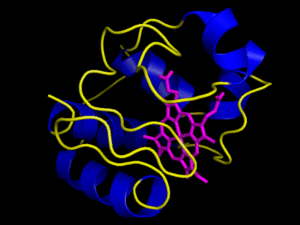 The heme group is a rigid carbon and nitrogen structure, called a porphyrin ring, surrounding a reducible iron atom. In the above picture, iron is in orange, nitrogen in blue, carbon in green and oxygen in red. While the structure pictured is typical of heme groups, the heme groups held by different cytochromes vary slightly, generally in their conformation. The hold the heme group in specific ways that slightly changes their conformations. This difference in the way a group is held yields the difference in redox potential that allows the Electron Transport Chain to have directionality, i.e. changes in the conformation of the prosthetic group yield differences in the group’s affinity for electrons. For example, the way in which cytochrome c holds its heme group is visualized in the animation to the right. However, the heme can be held in other ways, as visualized by the next image below it, the cytochrome bc complex. The cytochrome bc complex is made up of multiple proteins, many of which have their own prosthetic groups. Several of these prosthetic groups are heme groups, and a few are of the flavin type.
The heme group is a rigid carbon and nitrogen structure, called a porphyrin ring, surrounding a reducible iron atom. In the above picture, iron is in orange, nitrogen in blue, carbon in green and oxygen in red. While the structure pictured is typical of heme groups, the heme groups held by different cytochromes vary slightly, generally in their conformation. The hold the heme group in specific ways that slightly changes their conformations. This difference in the way a group is held yields the difference in redox potential that allows the Electron Transport Chain to have directionality, i.e. changes in the conformation of the prosthetic group yield differences in the group’s affinity for electrons. For example, the way in which cytochrome c holds its heme group is visualized in the animation to the right. However, the heme can be held in other ways, as visualized by the next image below it, the cytochrome bc complex. The cytochrome bc complex is made up of multiple proteins, many of which have their own prosthetic groups. Several of these prosthetic groups are heme groups, and a few are of the flavin type.
 The heme group is thus only one type of functional group that an electron carrier can have. Ubiquinone carries electrons by reducing two oxygens on a six membered carbon ring. This reduction of the oxidation causes the six membered ring to become aromatic. This gain of aromaticity will be one factor causing Coenzyme Q to be a better receptor of electrons than a carrier which lost aromaticity when reduced, such as NAD+. NAD+ has an aromatic pyridine ring, which when reduced gains an H and two electrons on the carbon opposite its Nitrogen. This addition causes the aromaticity of NAD+ to be lost. Like Ubiquinone, this loss in aromaticity is one of the factors which affect NAD+’s affinity for electrons. However in this case, it will decrease the molecule’s redox potential.
The heme group is thus only one type of functional group that an electron carrier can have. Ubiquinone carries electrons by reducing two oxygens on a six membered carbon ring. This reduction of the oxidation causes the six membered ring to become aromatic. This gain of aromaticity will be one factor causing Coenzyme Q to be a better receptor of electrons than a carrier which lost aromaticity when reduced, such as NAD+. NAD+ has an aromatic pyridine ring, which when reduced gains an H and two electrons on the carbon opposite its Nitrogen. This addition causes the aromaticity of NAD+ to be lost. Like Ubiquinone, this loss in aromaticity is one of the factors which affect NAD+’s affinity for electrons. However in this case, it will decrease the molecule’s redox potential.
Other prosthetic functional groups that exist include Flavin Mononucleotide, FAD, Copper Groups and Iorn-Sulfur clusters. These different prosthetic groups have different ways of carrying the electrons than heme groups, but are similarly used. The different prosthetic groups also have different affinities for electrons than each other, and by varying the conformation and type of prosthetic group that is held by a protein, a directional chain can be created to carry an electron from a high energy state to its lower energy state, releasing its energy in a controlled an productive way. In general cytochrome proteins and their heme groups have a higher affinity for electrons than Iron Sulfur groups. Thus, cytochrome carriers tend to be used at the end of the chain, and Iron sulfur at the beginning.
The information for this post was found in Albert’s Textbook.
13789hj said,
October 4, 2009 at 11:02 pm
this doesnt help
Elise Broach said,
December 13, 2009 at 2:47 pm
this is confusing and boring.
if you want to inform look at it from a students perspective. We try night and day to learn everything that the teachers are teaching us. So making a website colorful and interesting and EASY TO UNDERSTAND is better. make comparisons like photosynthesis is like the plant eating, or something similar. We need that extra push so help a little! make this easy to understand to a freshman in biology.
thank you!
Joe Wright said,
January 5, 2010 at 5:33 pm
This was not helpful at all. It taught me random things that i didnt need to know. This doesnt teach about NADH+ and confused me more than it helped. If it added uses of carrier molecules it would help more and make it seem more useful.
Celia said,
October 26, 2010 at 2:05 pm
Agreed. This didn’t help me. I was looking for the an easy to understand description of electron carrier molectules such as NAD, FAD, and NADP, but this article was just a bunch of irrelevant information.
donaisha said,
October 29, 2012 at 11:05 am
lame!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!